Immunoglobulin Structure and Classes
Immunoglobulins, also known as antibodies, are glycoprotein molecules produced by plasma cells or white blood cells. They specifically recognize and bind to particular antigens. This page introduces the nomenclature and criteria used to describe the structure, classes, and functional types of immunoglobulins.
Structure of immunoglobulins
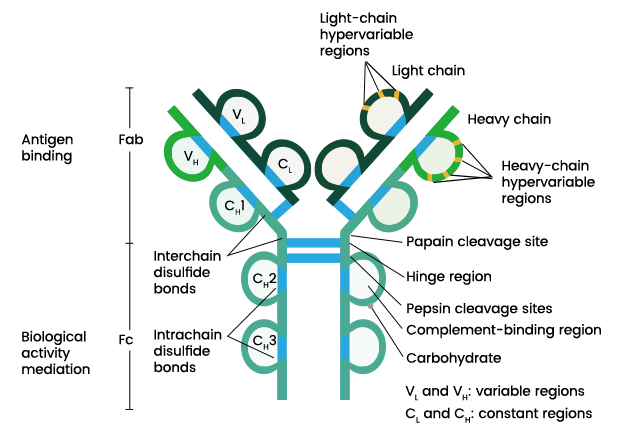
Antibody (or immunoglobulin) molecules are glycoproteins composed of one or more units, each containing four polypeptide chains: two identical heavy chains (H) and two identical light chains (L). The amino terminal ends of the polypeptide chains show considerable variation in amino acid composition and are referred to as the variable (V) regions to distinguish them from the relatively constant (C) regions. Each L chain consists of one variable domain, VL, and one constant domain, CL. The H chains consist of a variable domain, VH, and three constant domains CH1, CH2 and CH3. Each heavy chain has about twice the number of amino acids and molecular weight (~50,000) as each light chain (~25,000), resulting in a total immunoglobulin monomer molecular weight of approximately 150,000.Heavy and light chains are held together by a combination of non-covalent interactions and covalent interchain disulfide bonds, forming a bilaterally symmetric structure. The V regions of H and L chains comprise the antigen-binding sites of the immunoglobulin (Ig) molecules. Each Ig monomer contains two antigen-binding sites and is said to be bivalent.
The hinge region is the area of the H chains between the first and second C region domains and is held together by disulfide bonds. This flexible hinge (found in IgG, IgA, and IgD, but not IgM or IgE) region allows the distance between the two antigen-binding sites to vary.Heavy and light chains are held together by a combination of non-covalent interactions and covalent interchain disulfide bonds, forming a bilaterally symmetric structure. The V regions of H and L chains comprise the antigen-binding sites of the immunoglobulin (Ig) molecules. Each Ig monomer contains two antigen-binding sites and is said to be bivalent.
The hinge region is the area of the H chains between the first and second C region domains and is held together by disulfide bonds. This flexible hinge (found in IgG, IgA, and IgD, but not IgM or IgE) region allows the distance between the two antigen-binding sites to vary.
Classes of immunoglobulins
The five primary classes of immunoglobulins are IgG, IgM, IgA, IgD, and IgE. These are distinguished by the type of heavy chain found in the molecule. IgG molecules have heavy chains known as gamma-chains; IgMs have mu-chains; IgAs have alpha-chains; IgEs have epsilon-chains; and IgDs have delta-chains.
Differences in heavy chain polypeptides allow these immunoglobulins to function in different types of immune responses and at particular stages of the immune response. The polypeptide protein sequences responsible for these differences are found primarily in the Fc fragment. While there are five different types of heavy chains, there are only two main types of light chains: kappa (κ) and lambda (λ).
Antibody classes differ in valency as a result of different numbers of Y-like units (monomers) that join to form the complete protein. For example, in humans, functioning IgM antibodies have five Y-shaped units (pentamer) containing a total of ten light chains, ten heavy chains, and ten antigen-binding.
IgG class
Properties of IgG:
- Molecular weight: 150,000 Da
- H-chain type (MW): gamma (53,000 Da)
- Serum concentration: 10 to 16 mg/mL
- Percent of total immunoglobulin: 75%
- Glycosylation (by weight): 3%
- Distribution: intra- and extravascular
- Function: secondary response
IgM class
Properties of IgM:
- Molecular weight: 900,000 Da
- H-chain type (MW): mu (65,000 Da)
- Serum concentration: 0.5 to 2 mg/mL
- Percent of total immunoglobulin: 10%
- Glycosylation (by weight): 12%
- Distribution: mostly intravascular
- Function: primary response
IgA class
Properties of IgA:
- Molecular weight: 320,000 Da (secretory)
- H-chain type (MW): alpha (55,000 Da)
- Serum concentration: 1 to 4 mg/mL
- Percent of total immunoglobulin: 15%
- Glycosylation (by weight): 10%
- Distribution: intravascular and secretions
- Function: protect mucus membranes
IgD and IgE class
Properties of IgD:
- Molecular weight: 180,000 Da
- H-chain type (MW): delta (70,000 Da)
- Serum concentration: 0 to 0.4 mg/mL
- Percent of total immunoglobulin: 0.2%
- Glycosylation (by weight): 13%
- Distribution: lymphocyte surface
- Function: unknown
Properties of IgE:
- Molecular weight: 200,000 Da
- H-chain type (MW): epsilon (73,000 Da)
- Serum concentration: 10 to 400 ng/mL
- Percent of total immunoglobulin: 0.002%
- Glycosylation (by weight): 12%
- Distribution: basophils and mast cells in saliva and nasal secretions
- Function: protect against parasites
- Introduction to Antibody Production and Purification
- The ability of animal immune systems to produce antibodies capable of binding specifically to antigens can be harnessed to manufacture probes for detection of molecules of interest in a variety of research and diagnostic applications. No other current technology allows researchers to design and manufacture such highly specific molecular recognition tools.
- Nearly all medical or cell biology researchers doing any kind of molecular analysis make use of antibody technology in one form or another. Depending upon their specific research needs, scientists will differ in the extent to which they concern themselves with antibody production and purification.